Assay to measure direct factor Xa inhibitors in blood and plasma
Tech ID
18-045
Inventors
P. Y. Kim
C. Wu
Patent Status
US Patent 11034993
Stage of Research
Proof of principle data available
Contact
Sunita Asrani
Associate Director
Business Development and Copyright
Abstract
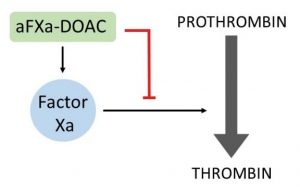
Anti-factor Xa direct oral anticoagulants (aFXa-DOACs), such as rivaroxaban and apixaban, represent a new class of drug being used more frequently in the treatment and prevention of arterial and venous thrombosis. As such, it is becoming more important to be able to measure the levels of these agents in the blood of patients. However, there is currently no simple way to accurately quantify circulating levels of aFXa-DOACs, particularly at very low concentrations.
This technology provides an assay to quickly and conveniently determine levels of aFXa-DOACs directly in whole blood or plasma.
Applications
- Accurate quantitation of aFXa DOAC levels in the blood with wide-ranging sensitivity
- Preparation for surgery to prevent bleeding complications
- Continuous patient monitoring for compliance and/or effect checks at the point of care
Advantages
- Can test both plasma and citrated whole blood to allow for simple, on-demand usage in hospital labs
- Sensitivity can be tailored to measure near therapeutic levels or very low (below trough) residual levels of aFXa-DOACs

