Developing rapid, accurate and sensitive molecular systems for detecting pathogenic Candida species
Tech ID
18-010
Inventors
A. El Sheikha
J. Xu
Patent Status
Provisional patent filed
Stage of Research
Proof of principle data available
Contact
Leigh Wilson
Associate Director, New Ventures
Abstract
Invasive fungal infections such as invasive candidiasis and candidemia affect over 250,000 people annually and result in more than 50,000 deaths worldwide. The close relationships among the pathogenic Candida species creates challenges in diagnosing the specific cause of infection with currently available methods, which lack accuracy, reproducibility, sensitivity and reliability (e.g., T2Candida). Thus, novel detection methods are needed to successfully diagnose the cause of infection and reduce unnecessary side effects, hospital expenditures and deaths.
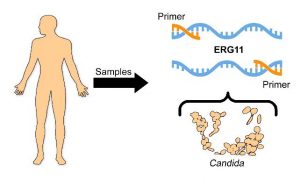
The present invention provides a reliable, accurate, reproducible, and sensitive rapid polymerase chain reaction (PCR) (regular and real-time PCR) technique for species-specific detection. This can aid in the diagnosis, identification, or monitoring of the progress of therapy for diseases caused by Candida species. This technique uses custom-designed species-specific biomarkers targeting the fungus specific ERG11 gene of the Candida species, which can be used to detect and distinguish eight species of Candida from a variety of human biological samples including C. albicans, C. dubliniensis, C. parapsilosis, C. orthopsilosis, C. metapsilosis, C. glabrata, C. krusei, and C. tropicalis.
Applications
- Clinical detection of eight pathogenic Candida species using a rapid polymerase chain reaction (PCR) method (regular and real-time PCR).
- Candida detection in test samples from humans such as blood, blood derived samples, tissues, tissue-derived samples and other body fluids.
- Diagnosis kit development aiding in the identification and monitoring of disease progression.
- Using this invention, a diagnostic device could be developed with the capabilities to specifically and accurately diagnose the cause of Candida infections.
Advantages
- Effective differentiation and accurate identification of the Candida species among the commonly found medically important fungi compared to current methods.
- Reliable, accurate, reproducible and increased sensitivity compared to existing techniques.

