Plant-derived pharmacological chaperones for treatment of phenylketonuria (PKU)
Tech ID
15-066
Inventors
P. Britz-McKibbin
Patent Status
US Patent 10426751
EP Patent 3242658
Stage of Research
Proof of Principle data available
Contact
Sunita Asrani
Associate Director
Business Development and Copyright
Abstract
Pharmacological chaperones (PCs) are a promising strategy for the treatment of genetic disorders based on enzyme enhancement therapy, such as phenylketonuria (PKU). PKU is a common in-born error of amino acid metabolism that is related to more than 500 disease-causing mutations of phenylalanine hydroxylase (PAH) or by a defect in the synthesis or re generation of tetrahydrobiopterin (BH4). To date, lifelong dietary Phe restriction and BH4 supplementation are the only accepted treatment options for PKU patients. However, special low-protein diets can lead to malnutrition, psychosocial or neurocognitive complications due to poor compliance, while BH4 therapy is costly and only 20-30% of PKU patients are responsive.
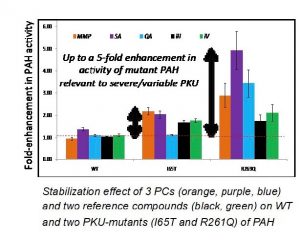
Using a novel screening strategy to identify small molecules from a chemical library with chaperone activity, McMaster researchers have identified plant-derived natural products (and synthetic analogs) that enhance the activity of denatured/inactive wild-type PAH and two clinically relevant PKU mutant enzymes. These plant-derived natural products are present in variable amounts in the human diet and thus offer a safe yet effective therapeutic treatment of PKU via nutritional supplementation, notably for patients with severe phenotypes.
Applications and Advantages
- Enzyme replacement therapy has recently been shown to reduce plasma Phe levels by 40% in mouse models of PKU. However recombinant enzyme therapy is costly and requires careful monitoring to avoid toxicity and immunogenicity.
- Only a handful of studies have identified putative PCs to correct the folding of PKU mutants and most of these have had only modest efficacy in enhancing mutant PAH activity.
- The current PCs can be administered orally and are safe natural products already present in the human diet. They offer a simple way to rescue mutant PAH from cellular degradation in vivo thus enabling rapid clinical translation. An unprecedented 5-fold enhancement in mutant PAH activity has been demonstrated with lead PC candidates.

