Treatment of Breast Cancer
Dronedarone & related compounds | Class III anti-arrhythmic drug | Drug re-purposing
Tech ID
15-060
Inventors
K. Jerzak
S. Dhesy-Thind
A. Bane
J. Cockburn
Patent Status
US Patent 10576056
EP Patent 3393466
Stage of Research
Proof of Principle data available
Contact
Sunita Asrani
Associate Director
Business Development and Copyright
Abstract
Effective and well-tolerated novel therapies are needed for the treatment of breast cancer and other cancers. Unfortunately, the length of time required to develop a new drug is increasing, averaging approximately 14 years and costing US$1.8 billion. This long development period and high cost of new oncology drugs is largely due to unexpected problems once they reach testing in human clinical trials. Although drugs may appear to be effective in cell cultures and/or animal models, they often cause unacceptable toxic side effects in humans.
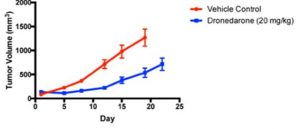
Dronedarone is an FDA-approved oral drug that is used to treat patients with cardiac arrhythmias but has shown to anti-cancer activity in breast cancer cell cultures, sphere forming assays and in mice. Given its established safety profile and pre-clinical efficacy data, Dronedarone offers a tantalizing opportunity for drug re-purposing as a potential anti-cancer agent, primarily through its effects on the induction of apoptosis and MYC inhibition.
Scientists at McMaster have shown convincing data demonstrating that Dronedarone and related compounds (which are structurally similar and likely to have comparable safety profiles) hold promise as novel anti-cancer agents for the treatment of patients with breast and other cancers.
Applications and Advantages
- Novel, oral anti-cancer agent for breast cancer and other cancers
- Established safety profile with FDA approval for an anti-arrhythmic indication

